Information on Injecting PrNUCEIVATM
This content has been created for HCPs.
For further information, please contact nuceivainfo@clarionmedical.com
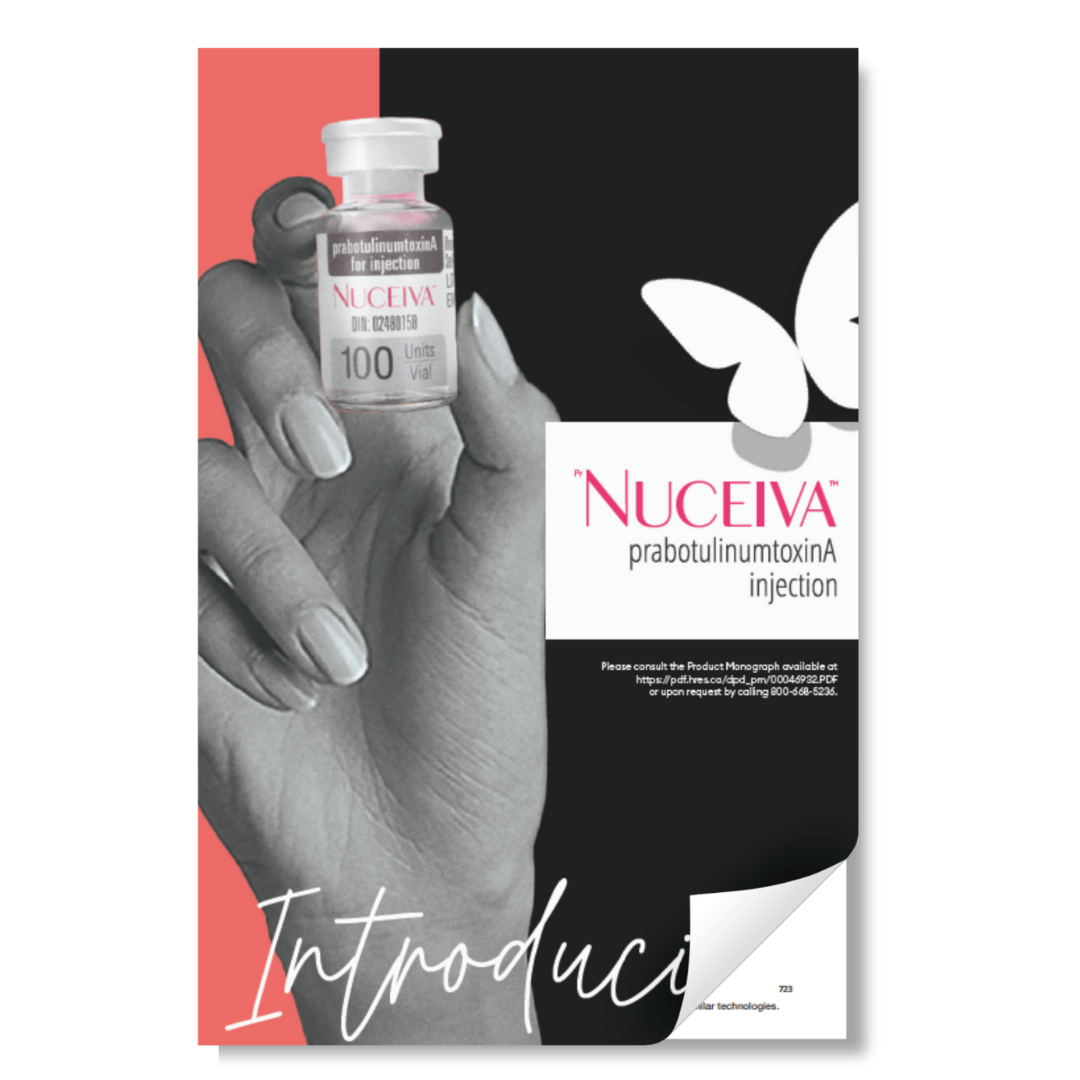
Indications and Usage
PrNUCEIVATM is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines in adult patients < 65 years of age.



.png)
.jpg)
.png)
.png)
.png)
.png)
.png)
.png)

.png)
-3.png)
-Dec-10-2024-07-21-37-0575-PM.png)

.png)
.png)

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)




